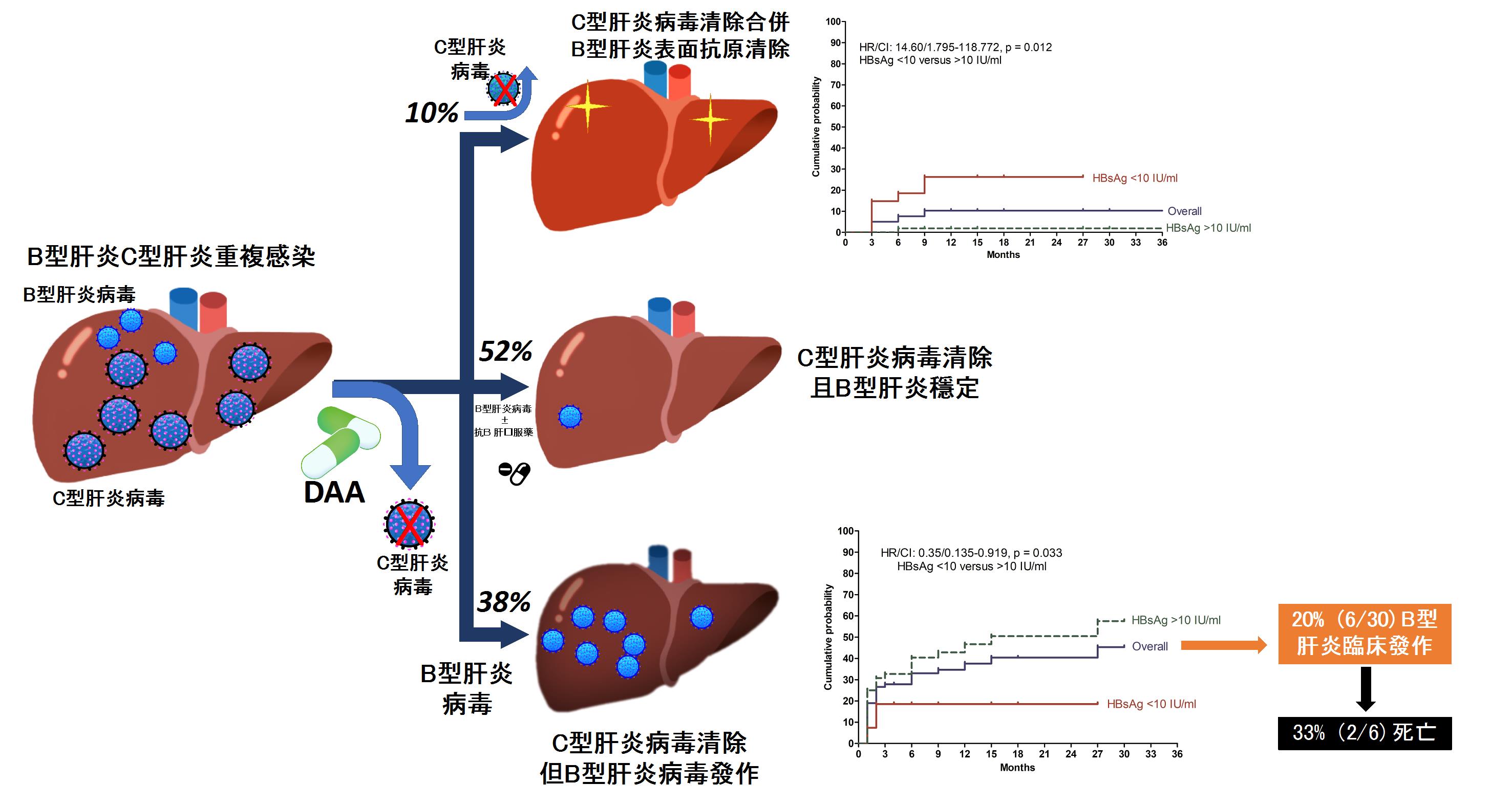
慢性B型與C型肝炎雙重感染病患接受口服抗病毒藥物成功治療C型肝炎後,可能引發B型肝炎嚴重發作及死亡
慢性B型與C型肝炎雙重感染常會加重C型肝炎之嚴重度並加速病程惡化。新型C型肝炎口服抗病毒藥物(DAA)上市後,可以有效清除95%以上之C型肝炎病毒,然而其對合併之B型肝炎感染之長期預後仍不得而知。高醫肝炎團隊針對台灣慢性B型與C型肝炎雙重感染病患接受C型肝炎口服抗病毒藥物治療後對B型肝炎相關預後及機轉進行研究,研究顯示有高達38%慢性B型與C型肝炎雙重感染病患接受DAA治療後,發生B型肝炎病毒發作,其中五分之一出現臨床肝炎發作,當中又有三分之一導致死亡。然而,也有10%病患在接受DAA治療後B型肝炎表面抗原消失,代表病患由B型肝炎帶原者轉變成非帶原者。我們進一步發現, 使用B型肝炎表面抗原濃度(HBsAg),以10 IU/ml分水嶺,可以成功預測病人未來會發生B型肝炎病毒發作(>10 IU/ml, (hazard ratio [HR] 2.88; 95% CI 1.057–7.844)或者是B型肝炎表面抗原消失(。這些發現顯示,治療前B型肝炎表面抗原濃度可以作為預測這類病患接受DAA治療是否能產生B型肝炎表面抗原消失及發生B型肝炎病毒發作的標記,近一步幫助指引臨床醫師治療此類病患前是否給予預防性B型肝炎抗病毒藥。我們的研究報告中另一重要發現是這些接受DAA治療後發生B型肝炎發作導致死亡的病患都是肝硬化同時沒有接受預防性抗B型肝炎藥物之病患,因此未來應考慮對肝硬化病患同時給予預防性B型肝炎抗病毒藥以減少風險。
除此之外,本研究也進行一系列細胞激素與微核醣核酸檢測來探討兩種病毒間免疫反應。 我們發現,治療前血清miR-122及miR-125b濃度較低、或治療過程當中血清IP-10濃度沒有降低的病人,B型肝炎表面抗原消失的機會明顯較高;相對的,B型肝炎病毒發作的病人,其治療過程當中血清miR-122及miR-125b濃度有持續降低的現象。
本研究結果可作為臨床醫師在診療此類病患之參考,幫助指引臨床醫師治療此類病患前是否給予預防性B型肝炎抗病毒藥以避免治療過程中可能發生的B型肝炎嚴重發作及死亡。同時減少無或微風險病患可能接受預防性B型肝炎抗病毒藥物之副作用風險與醫療花費。
本校主要研究者之簡介:
葉明倫副教授和余明隆講座教授為高雄醫學大學醫學院肝炎研究中心成員,也是高雄醫學大學附屬機構肝膽胰內科的主治醫師,致力於肝臟疾病之病因、致病機轉、治療及預防的研究及服務。
研究聯繫Email:
fish6069@gmail.com; fishya@ms14.hinet.net
期刊出處:
Yeh ML, Huang CF, Huang CI, Holmes JA, Hsieh MH, Tsai YS, Liang PC, Tsai PC, Hsieh MY, Lin ZY, Chen SC, Huang JF, Dai CY, Chuang WL, Chung RT, Yu ML*. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection. J Hepatol. 2020 Jul;73(1):62-71.
研究全文下載:
Taiwanese patients with HBV and HCV co-infection are likely to experience HBV functional cure, but also HBV reactivation following direct-acting antiviral treatment for HCV
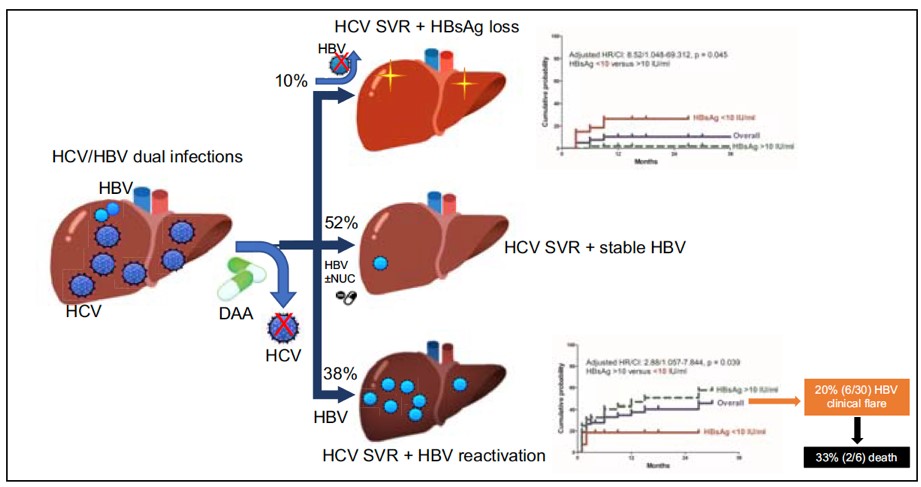
This study shows the outcomes after the direct acting antiviral (DAA) treatment in patients with chronic hepatitis B (HBV)/ hepatitis C (HCV) dual-infection, that including 10% of hepatitis B surface antigen (HBsAg) loss and 38% of HBV reactivation. Our findings published in the Journal of Hepatology provide new insights into understanding the viral interaction between HBV and HCV.
The research group led by Chair Professor Ming-Lung Yu from Hepatitis Research Center, Kaohsiung Medical University, Kaohsiung, Taiwan, in collaboration with Professor Raymond T Chung from Massachusetts General Hospital, Boston; Department of Medicine, Harvard Medical School, reported a new insight into the viral interaction between HBV and HCV following DAA treatment and found it might be a blessing (HBsAg loss) in disguise (HBV reactivation) for patients. They also suggested the high risked cirrhotic patients to undergo HBV prophylaxis simultaneously.
The new all-oral DAA regimens for HCV have greatly improved treatment efficacy with sustained virological response rates of > 95% for HCV monoinfected patients. Unfortunately, HBV reactivation and HBV-related clinical reactivation during or after DAA therapy were not infrequent, and might lead to mortality in HBV/HCV-coinfected patients, especially among those with cirrhosis.
This study enrolled 79 HBV/HCV-coinfected patients receiving DAA therapy to investigate their HBV-related outcomes. One exciting finding was that during one year post-DAA follow up, 8 (10.1%) patients experienced HBsAg loss (an ideal outcome of “HBV functional cure”) with a 12-month cumulative probability of 10.3%. Another new finding was the specific decline of HBsAg level during therapy, and rebound after stopping DAA.
Alternatively, up to 30 (38%) patients were found developing a HBV reactivation (means a rebound of HBV replication). Of them, 6 (20%) patients even experienced a HBV related clinical reactivation (means a clinical hepatitis) and 3 patients further developed hepatic failure with 2 died despite the immediate anti-HBV therapy. All these 2 patients had liver cirrhosis and were without an HBV prophylaxis before DAA. Thus prophylactic anti-HBV therapy is mandatory for cirrhotic patients, irrespective of baseline HBV DNA levels.
Pre-treatment HBsAg titer can be a surrogated marker to guide decision making. This study further found that using the pre-treatment HBsAg titer of less than 10 IU/mL could predict the higher chance of HBsAg loss and lower risk of HBV reactivation.
Researchers from Kaohsiung Medical University and Harvard Medical School also demonstrates a complex interaction among the host and the two viruses in determining HBV outcomes following the results from cytokines and micro RNAs experiments.
Getting together, this study provides a new insight into the management of HBV/HCV-coinfected patients being treated with DAA.
Main researcher Intro.
Associate Professor Ming-Lun Yeh and Chair Professor Ming-Lung Yu are members of Hepatitis Center and Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University Hospital and Hepatitis Research Center, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan. They dedicate in the research and service of Liver diseases, including etiologies, epidemiology, pathogenesis, therapeutic modalities and prevention of hepatitis B, hepatitis C, steatohepatitis and liver cancers.
Author Email
fish6069@gmail.com; fishya@ms14.hinet.net
Paper cited from:
Yeh ML, Huang CF, Huang CI, Holmes JA, Hsieh MH, Tsai YS, Liang PC, Tsai PC, Hsieh MY, Lin ZY, Chen SC, Huang JF, Dai CY, Chuang WL, Chung RT, Yu ML*. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection. J Hepatol. 2020 Jul;73(1):62-71.
Research Paper available online on website

