應用藍光LED來合成具藥物活性的骨架
簡介:
大家耳熟能詳的藍光LED常見於做為高效率燈泡,高醫大研究團隊開發以藍光照射引起自由基級聯環化反應以合成吡咯烷(一種藥物骨架)。該反應以極其溫和的室溫條件下,用藍光LED照射1,6-烯炔類反應物,進行環化產生三個新的化學鍵形成吡咯烷。本方法具有極高的位向和化學選擇性,更重要是原子經濟,綠色又環保,是合成藥物新方向。具有吡咯烷骨架的藥物有:Nicotine、 Captopril (抗高血壓藥)、NK3 antagonist (巴金森症)、TACE inhibitor (抗癌藥) 等等,希望這方法可做為相關藥物合成新選擇。
圖形摘要:
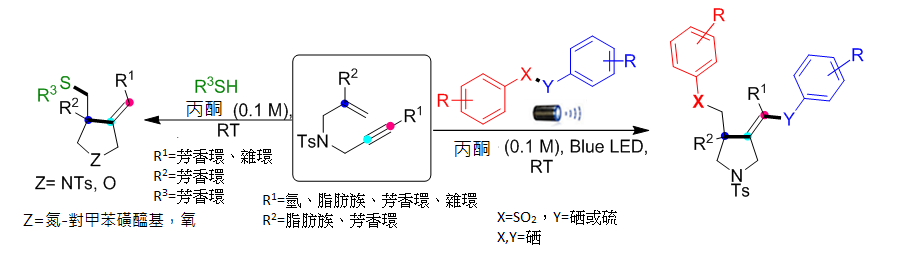
1.
以藍光LED照射合成吡咯烷,此類化合物結構常見於諸多藥物。
2.
本方法具有極高的位向和化學選擇性,更重要是原子經濟,綠色又環保,是合成藥物新方向。
3.
反應的官能基耐受性廣泛且為環境友善的條件,符合永續的概念。
【研究團隊】
團隊成員:王志鉦教授、Mohana
Reddy Mutra博士
代表單位:高雄醫學大學醫藥暨應用化學系
團隊簡介:高雄醫學大學醫藥暨應用化學系 王志鉦 教授
電話:+ 886-7-3121101分機2275,電子郵件:jjwang@kmu.edu.tw
高雄醫學大學醫藥暨應用化學系
Mohana Reddy Mutra 博士後研究員
電話:+886-7-3121101分機2275,電子郵件:mohan.mohan2060@gmail.com
【論文資訊】
論文出處:Green Chem., 2020, 22, 2288-2300
全文下載:https://pubs.rsc.org/en/content/articlehtml/2020/gc/d0gc00321b
Alkene versus alkyne reactivity in unactivated
1,6-enynes: regio- and chemoselective radical cyclization with chalcogens under
metal- and oxidant-free conditions
Abstract:
Herein, we have developed metal and oxidant-free visible
light-promoted alkene vs. alkyne regio- and chemoselective radical cascade
cyclization of electronically unbiased 1,6-enynes with chalcogens to synthesize
substituted pyrrolidines bearing chalcogens. The reaction generated three new
bonds, namely, C–SO2, C–C, and C–Se under extremely mild conditions.
Furthermore, we achieved regio- and chemoselective mono-addition of aromatic
thiophenols with unactivated 1,6-enynes.
Graphical Abstract
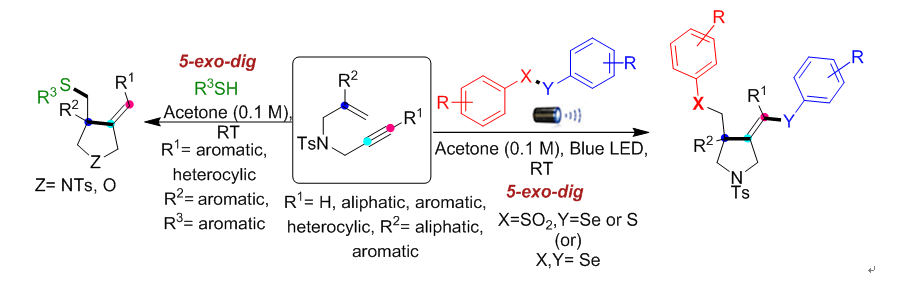
Application and Highlights:
1. Pyrrolidine is a versatile
structural scaffold with diverse pharmacological applications and it exists in
many natural products introducing the chalcogen functionality into the pyrrolidine
skeleton may provide an opportunity to enhance the reactivity of native
compounds or drugs.
2. Metal
and oxidant-free mild conditions of visible light-promoted radical cascade
cyclization.
3. The
key features of this protocol are broad substrate scope, environment-friendly
conditions, operational simplicity, atom economy, and amenability to gram-scale
synthesis. The mechanistic studies corroborate that the reaction proceeds via a
radical pathway.
Research Team Members:
Dr. Jeh-Jeng Wang and Mohana Reddy Mutra
Representative Department: Department
of Medicinal and Applied Chemistry, Kaohsiung Medical University
Introduction of Research Team:
Dr. Jeh-Jeng Wang, Professor, Department of Medicinal and Applied Chemistry, Kaohsiung Medical University. Tel: +886-7-3121101(Ext:
2275), E-mail: jjwang@kmu.edu.tw
Dr.
Mohana Reddy Mutra, Postdoctoral fellow, Department of Medicinal and Applied
Chemistry, Kaohsiung Medical University. Tel:
+886-7-3121101(Ext: 2275), E-mail: mohan.mohan2060@gmail.com
Contact
Email: jjwang@kmu.edu.tw
Publication: Green Chem.,
2020, 22, 2288-2300
Full-Text Article: https://pubs.rsc.org/en/content/articlehtml/2020/gc/d0gc00321b

